Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Elastic and Structural Studies on some Bismuth Borate Glasses using Ultrasonic and Spectroscopic Techniques
Authors: Sumathi T, Soundarraj C, Parkavi S
DOI Link: https://doi.org/10.22214/ijraset.2024.66018
Certificate: View Certificate
Abstract
B2O3-Bi2O3, B2O3-Bi2O3-ZnO and B2O3-Bi2O3-CaO glasses of different compositions were prepared by using the melt quench technique. The Ultrasonic velocities (both longitudinal and transverse) were measured at 10MHz at room temperature using the pulse-echo overlap method. From the measured values of velocity and density, elastic constants and other parameters such as molar volume, Poisson\'s ratio, acoustic impedance, microhardness, Debye temperature and thermal expansion coefficient have been evaluated. The variations in these parameters are analysed in terms of structural changes in the glass network with composition. The amorphous nature of the glass samples is studied using XRD. The FTIR investigation is used to identify the functional groups present in the glass samples.
Introduction
I. INTRODUCTION
Ultrasonic technique similar to other techniques plays a significant role in understanding the structural characteristics of glass networks. The acoustic wave propagation in bulk glasses has been of considerable interest to understand their mechanical properties. Elastic properties of solid materials are of considerable significance because their measurement yields information concerning the forces that operate between the atoms or ions comprising solids. This is fundamentally important in interpreting and understanding the native bonding in solid-state materials [1]. B2O3 is one of the most important glass formers incorporated into various kinds of glass systems as a flux material to attain materials having specific physical and chemical properties suitable for high technological applications. [2]. The boron atom in borate crystals and glasses usually coordinates with either three or four oxygen atoms forming BO3 or BO4 structural units [3]. It is well known that glasses that include bismuth oxide (Bi2O3) are considered promising heavy metal oxide glasses. This is because of their unique features such as their higher linear refractive index and their higher density when compared to other types of glasses. Bi2O3 is a basic glass former, thus, when it is applied to the glass composition, it affects the overall properties of the glass. The Bi2O3 glasses are important in different optical applications [4].
Glasses containing divalent ions such as Mg2+, Zn2+, Pb2+, Ca2+, etc play an important role both in the formation and modification of glass structure. The physical properties of borate glasses can be altered by the addition of a network modifier (alkali and alkaline earth oxides) [5]. It has been found that B2O3 could be used as a good network former (NWF) and other chemicals such as Bi2O3 and ZnO could be found as network modifiers (NWM) when those are added to the Bi2O3 content. It has also been noticed that with the presence of ZnO content in the glassy materials, the stability of the material becomes stronger with a high thermal resistance against the crystallization. Similar to Zinc oxide (ZnO), calcium oxide (CaO) has also been extensively used as a component ensuring an improvement of several physiochemical properties of glasses. Calcium oxide is widely used in industry, e.g., in making porcelain and glass; in purifying sugar, in preparing bleaching powder, calcium carbide and calcium cyanamide; in water softeners and mortars and cements. In agriculture, it is used for treating acidic soils(liming). A variety of glass systems have been investigated by many researchers [6],[7],[8]. Ultrasonic velocity measurements, yield information about elastic moduli, Poisson's ratio, acoustic impedance, thermal coefficient and Debye temperature. Despite the immense use of ultrasonic techniques in understanding the structure and properties of glasses, only a limited number of reports have appeared on borate-based glasses.
The present work aims at the preparation of the following three series of glasses namely BB (Binary), BBZ and BBC (Ternary) glass systems. These three glass systems are discussed by using Ultrasonic velocity, XRD and FTIR study.
B2O3 - Bi2O3 (BB)
B2O3 - Bi2O3 – ZnO (BBZ)
B2O3 - Bi2O3 – CaO (BBC)
II. MATERIALS AND METHODS
Two series of glasses, each containing 4 specimens are prepared from AR grade chemicals (minimum assay 99.9%) B2O3, Bi2O3, ZnO and CaO. The required amount (approximately 20g) in mol% of different chemicals in powder form was weighed using a single pan digital balance having an accuracy of ±0.0001g. The homogenization of the appropriate mixture of the component of chemicals is effected by repeated grinding using a mortar. The homogeneous mixture is put in a silica crucible and placed in a furnace. Melting is carried out under controlled conditions at a temperature from 900 to 960°C for all three systems. The molten sample is cast into a copper mould having dimensions of 10mm diameter and 6mm length. Then the glass samples are annealed at 400°C for two hours to avoid the mechanical strain developed during the quenching process. The samples prepared are chemically stable and non-hygroscopic. The prepared glass specimens are polished and the surfaces are made perfectly planed and smoothened by diamond disc and diamond powder. The thickness of the samples has been measured using digital vernier callipers with an accuracy of 0.0001mm. The nomenclature and composition of glass samples are given in Table. 1 and the photograph of the glasses in Plate 1 and 2.
Table 1 Nomenclature and composition of glasses
|
Sample No |
Nomenclature |
Composition in mol% |
Remarks |
|||
|
PLATE 1 1 2 3 4 |
BBZ5 BBZ10 BBZ15 BBZ20 |
70-25-5 70-20-10 70-15-15 70-10-20 |
Mol % of B2O3 is constant |
|||
|
PLATE 2 1 2 3 4 |
BBC5 BBC10 BBC15 BBC20 |
70-25-5 70-20-10 70-15-15 70-10-20 |
Mol % of B2O3 is constant |
|
||

PLATE - 1 PLATE - 2
A.Theory and Calculations
Various parameters of the glass specimen are calculated using the measured density (ρ), longitudinal velocity (Ul) and shear velocity (Us) using the standard expressions given below [9],[10].
Molar volume (Vm ) Vm = Mρ (1)
Longitudinal modulus (L) L= ρUl2 (2)
Shear modulus (G) G= ρUS2 (3)
Bulk modulus (K) K=L-43G (4)
Young’s modulus (E) E = (1 + σ) 2G (5)
Poisson’s ratio (?) σ= L-2G2(L-G) (6)
Acoustic impedance (Z) Z = ρ Ul (7)
Microhardness (H) H=(1-2σ)E6(1+σ) (8)
Debye Temperature (θD) θD= hK9N4πVm13Um (9)
Mean sound velocity (Um ) Um =132Us3+1Ul3-13 (10)
Thermal expansion coefficient (αp) αp = 23.2 (Ul – 0.57457) (11)
III. RESULTS AND DISCUSSION
The experimental values of density (ρ), longitudinal ultrasonic velocity (U1), shear-ultrasonic velocity (Us) and molar volume (Vm) of the different glass specimens concerning the change in the mol % of ZnO and CaO are reported in Table 1. Also the longitudinal modulus (L), shear modulus (G), bulk modulus (K), Young's modulus (E) and Poisson's ratio (?) of glass samples BB, BBZ and BBC are reported in Table 2.
The acoustic impedance (Z), microhardness (H), Debye temperature (?D) and thermal expansion coefficient (αp ) of glass samples BB, BBZ and BBC are presented in Table 3.
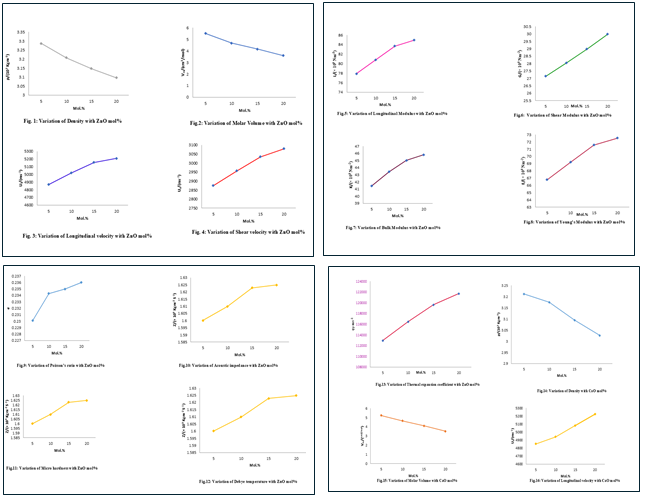
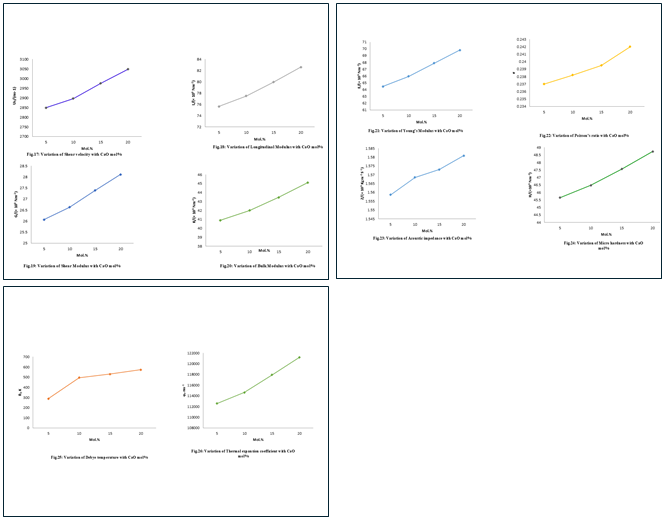
(Figs 1 to 13) show the variation of density, molar volume, longitudinal velocity, shear velocity, longitudinal, shear, bulk and Young's modulus, Poisson's ratio, acoustic impedance, microhardness, Debye temperature and thermal expansion coefficient in four BBZ glasses with composition of ZnO (mol%). Similarly, the variation of these parameters with the composition of CaO (mol%) of four BBC glasses is depicted in (Figs. 14 to 26).
The variation of density and molar volume is shown in Table 1. Density is the physical property of a material, which is an important tool capable of exploring the tightness and changes in the structure of the material. It is affected by the structural, softening, change in the geometrical configuration, coordination number, cross-link density and dimension of interstitial spaces in the glass. It has been observed from Table 1, that the density decreases linearly with the increase of ZnO and CaO content at the expense of Bi2O3 content. With the increase in the concentration of ZnO and CaO, the density of the glasses decreases, probably due to the size effect with the replacement of Bismuth oxide atoms (atomic mass 465.95 u) by the lighter zinc oxide (atomic mass-81.40 u) and calcium oxide (atomic mass-56.07 u) atoms [11],[12].
In short, the decrease in density of the glasses accompanying the addition of ZnO and CaO is probably attributed to a change in cross-link density and coordination numbers.
Table 2
Values of density (ρ), longitudinal velocity (Ul), shear velocity (Us) and molar volume (Vm) of BB, BBZ and BBC glass systems
|
Name of the Samples |
Density ρ/(x103 kg.m-3) |
Ultrasonic velocity (ms-1)
|
Molar volume (cm) cm3/mol |
|
|
Longitudinal (Ul)
|
Shear (Us) |
|||
|
BB |
3.3593 |
4754.00 2816.02 |
2.6351 |
|
|
BBZ glass system |
||||
|
BBZ5 BBZ10 BBZ15 BBZ20 |
3.2871 3.2074 3.1474 3.0971 |
4868.08 5019.49 5156.07 5209.29 |
2874.13 2956.93 3034.62 3079.48 |
5.5086 4.6787 4.1570 3.6036
|
|
BBC glass system |
||||
|
BBC5 BBC10 BBC15 BBC20 |
3.2119 3.1748 3.0946 3.0258 |
4852.73 4940.77 5083.18 5224.72 |
2848.67 2896.13 2974.97 3048.13 |
5.2314 4.6470 4.1051 3.5211 |
Table 3
Values of longitudinal, shear, bulk and Young’s moduli and Poisson’s ratio of BB, BBZ and BBC glass systems
|
Name of the Sample
|
Longitudinal modulus L/ (× 10 9N.m -2)
|
Shear modulus G/(× 10 9N.m-2)
|
Bulk modulus K/(×109N.m -2)
|
Young’s modulus E/(×109 N.m -2)
|
Poisson’s ratio (σ)
|
|||
|
BB |
75.9219 |
26.6391 |
40.40 |
65.62 |
0.2297 |
|||
|
|
BBZ glass system |
|||||||
|
BBZ5 BBZ10 BBZ15 BBZ20 |
77.8983 80.8113 83.6738 84.9509
|
27.1534 28.0436 28.9841 29.9841
|
41.46 43.42 45.03 45.81
|
66.79 69.21 71.58 72.55
|
0.2301 0.2343 0.2350 0.2360
|
|||
|
|
BBC glass system |
|||||||
|
BBC5 BBC10 BBC15 BBC20 |
75.6369 77.5007 79.9604 82.5973 |
26.0643 26.6288 27.3885 28.1129
|
40.88 41.99 43.44 45.11
|
64.45 65.94 67.89 69.82
|
0.237 0.2382 0.2395 0.2420
|
|||
Table 4
Values of acoustic impedance (Z), microhardness (H), Debye temperature (θD) and thermal expansion coefficient (αp) of BB, BBZ and BBC glass systems
|
Name of the Sample
|
Acoustic impedance Z/(×10 7kgm-2s-1)
|
Micro hardness H/(×10 9 Nm -2)
|
Debye temperature θ D K
|
Thermal expansion coefficient αpms-1
|
||||
|
BB |
75.9219 |
26.6391 |
40.40 |
65.62 |
||||
|
|
BBZ glass system |
|||||||
|
BBZ5 BBZ10 BBZ15 BBZ20 |
1.6001 1.6099 1.6228 1.6248
|
48.8486 49.6613 51.1975 51.6537
|
218.6614 470.9527 535.4762 567.3818
|
112926.126 116438.838 119607.494 121700.598
|
||||
|
|
BBC glass system |
|||||||
|
BBC5 BBC10 BBC15 BBC20 |
1.5586 1.5685 1.5730 1.5808
|
45.6549 46.4736 47.5604 48.7355
|
287.3877 494.4307 530.1285 573.4712
|
112570.006 114612.534 117916.446 121200.174
|
||||
Molar volume (Vm) (Figs. 2 and 15) which is defined as the volume of one gm mole of glass decrease from 5.5086X 10-5 to 3.6036 x 10-5 m3mol-1 for BBZ and 5.2314 X 10-5 to 3.5211 X 10- 5 m3 mol -l for BBC systems as the ZnO and CaO content increase (Table 2). The glass structure is more explained using molar volume than density, as the former has to do with ions spatial distribution in structure. The molar volume changes with the ZnO and CaO content. The molar composition is an indication of the preceding structural changes via a modification or formation process in the network of the glass [13].
Ultrasonic velocities both longitudinal and shear velocities increase with the increase in mol% of ZnO and CaO content (Figs. 3 to 17). One can notice from Table 3 that the longitudinal (Ul) and shear velocities (Us) increase linearly with an increase in mol% B2O3 in both BBZ and BBC glass systems. It is observed that the rate of increase of Ul is greater than that of Us. Also, it is observed that the longitudinal wave velocity is greater than that of the shear wave velocity. This is due to the particles oscillating parallel to the direction of propagation for shear waves [14]. The increase of ultrasonic velocity both longitudinal and shear is attributed to an increase in the rigidity of the glass network structure [15].
The calculated elastic moduli such as longitudinal modulus (L), shear modulus (G), bulk modulus (K) and Young's modulus (E) are reported in Table 4. The longitudinal, shear, bulk and Young's moduli increase in the entire range of composition in both the glass systems. The increase of elastic moduli is due to the increase in creating bridging oxygen atoms. The increase in the values of the elastic constants has been attributed to an increase in the packing density and rigidity and hence the formation of stronger structural building units in the glass network. In general, the addition of ZnO and CaO to a bismuth borate glass network increases the rigidity, the velocity and hence the elasticity of the glass.
Poisson's ratio (σ) (Figs. 9 and 26) can be determined from the ultrasonic wave velocities. Poisson's ratio (σ) is the ratio of the transverse and linear strains for a linear stress and depends on both the dimensionality of the structure and cross-link density. The values of Poisson's ratio were found to increase from 0.2301 to 2360 for BBZ and 0.237 to 0.2420 for BBC glass systems [16], [9]. The increase in Poisson's ratio shows that the atoms experience higher transverse contraction strain action on them and hence become more tightly packed. A decrease in cross-link density leads to an increase in Poisson's ratio of atoms or ions [17]. In Table 3 and 4, the acoustic impedance increases with an increase in mol % of ZnO and CaO content in the glass system confirming the increase in rigidity of the structure of the glass. A similar trend is obtained by Sangeetha et al., [18].
X-ray diffraction is the most used investigation method to establish if a material is crystalline, vitreous or amorphous, Fig. 27 shows the X-ray diffraction pattern (XRD) of BB, BBZ5 and BBC5 glass specimens. The XRD spectrogram shows a broad hump which is characteristic of amorphous structure. The XRD spectrogram of BB, BBZ5 and BBC5 glass samples does not demonstrate any detectable peak and the patterns indicate that glass samples have pure amorphous, Non-crystalline structure.

Fig. 27. XRD pattern of BB, BBZ5 and BBC5 glass specimen
FTIR transmittance spectra of BB, BBZ5 and BBC5 glasses in the frequency range between 400 and 4000 cm-1 are shown in (Figs. 28 and 29). The broadening of IR bands indicated that there is no detectable change due to crystallization. The observed bands along with their vibrational assignments of samples been tabulated in Table 5.
The vibrational modes of the borate network are active mainly in three regions.
- The first region lies between 600 and 800 cm - 1 and is due to bending vibration of various borate segments.
- The second region lies between 850 – 1200 cm- 1 and is due to stretching vibrations of tetrahedral BO4 units.
- The third region lies between 1200 and 1600 cm- 1 and is due to stretching vibrations of B-O in BO3 triangles.
The present FTIR spectra showed the non-existence of a band at 806 cm-1. This reveals the absence of boroxol rings in glasses and hence it consists of only BO3 and BO4 groups [19]. The band has been observed in 686-694 cm- 1 spectral region is assigned to B-O-B bending vibrations in [BO3] triangles. The bands 870 – 1020 cm- 1 can attributed to B-O stretching vibrations of BO4 tetrahedral units The band in the region 1242- 1383 cm- 1 are attributed to the vibration in the units [16]. Bi2O3-containing glasses have fundamental vibrations in the IR spectral regions centred at 400-600 cm-1 characteristics to the Bi-O-Bi and Bi-O bonds vibrations in BiO6 units The obtained band from 408 – 485 cm- 1 can be ascribed to the vibrations of Bi-O-Bi and Bi-O bonds in [BiO6] octahedral units [15]. The bands from 484 – 488 cm- 1 are due to the Ca-O bending vibrations in the CaO units [20]. Also, its intensity increases with the increase in the content of modifier oxide, which is due to the formation of BO3 units at the expense of BO4 units. B2O3 is a network former with B2O3 and BO4 structural units. CaO when incorporated into a B2O3 glass network, normally converts into more stable BO4 units and may also create non-bridging oxygen (depending upon whether CaO goes into modifying positions or network-forming positions). The spectral region around 419-424 cm-1 is due to the vibration of cations such as Zn [21],[22] and hence network-modifying behaviour is observed in which these ions enter the interstices of the network. In addition to the above features, a transmittance band appears in the spectra of all glasses around 3500 cm¹, such as the band is attributed to the stretching vibration of -OH ion.
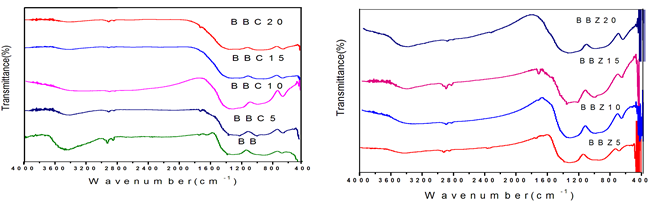
Fig.28 FTIR spectra of BBC glass specimen Fig.29 FTIR spectra of BBZ glass specimen
Tables.5:
FTIR tentative frequency assignment of BBZ and BBC Glasses
|
S.No
|
Wave Numbers (cm- 1)
|
Band Assignments |
References |
|
1 |
686-694
|
B-O-B bending vibrations in [BO3] triangles |
Abdelazis et al., 2014.
|
|
2 |
870-1020
|
Symmetric stretching vibrations of BO4 units |
Limkitjaroenporn et al.,2011. |
|
3 |
1242-1383
|
Stretching vibrations of B-O of trigonal (BO3)3- units |
Yasser B Saddeek and M.S.Gaafar,2009. |
|
4 |
408-485
|
Bi-O- Bi + Bi-O in BiO6 octahedral |
Ardeleamet al., 2005, E.M.Lineset al., 1987. |
|
5 |
484-488
|
Ca-O bending vibrations in the CaO units |
Pascuta, 2010.
|
|
6 |
419-424
|
Zn-O tetrahedral bending vibrations of ZnO4 units |
Del Longo et al., 1998, Motkeet al., 2002. |
Conclusion
From the present investigation, the following conclusions have been drawn on BB, BBZ and BBC glass systems using Ultrasonic, FTIR and XRD studies.XRD patterns predict the nature of samples as amorphous. The gradual decrease in density with mol% of ZnO and CaO of the glass specimen indicates the dependence of density on the weight of the metal atom in the network modifier. The Ultrasonic velocities (both longitudinal and shear) of both systems vary linearly and the magnitude is in the order BBZ > BBC. The estimated elastic moduli, acoustical and mechanical properties of the glasses throw light network. The rigidity and compactness in structural-functional groups present in the glass samples have been confirmed by FTIR spectral analysis.
References
[1] A. B. Vikas Veeranna, K. Sandip, A. Zalawadiya , “Comparative analysis of red cell distribution width and high sensitivity C-reactive protein for coronary heart disease mortality prediction in a multi-ethnic population”. journal-of-cardiology., vol. 168, pp. 5156-5161, 2013. [2] M.Yano, S. Kobayashi , “FKBP12.6-Mediated Stabilization of Calcium-Release Channel (Ryanodine Receptor) as a Novel Therapeutic Strategy Against Heart Failure”. Journal of AHAIASA journals., vol. 107, pp. 477- 484, 2003. [3] E. Abou Saleh, B. Yasser, “Does intravenous contrast agent affect dose calculations of three-dimensional treatment planning system”. Journal of Biblioteca virtual em saude., vol. 45(1), pp. 103-108, 2009. [4] S. D. Bale, S. T. Badman, J. W. Bonnell, “Highly structured slow solar wind emerging from an equatorial coronal hole”. Vol. 576, pp.237-242, 2019. [5] M . Ernest levin, “The System Bi2O-B2O3”. Journal of American Ceramic Society., vol. 45, pp. 355-360, 1962. [6] R. El-Mallawany, M.I. Sayyed,” Simulation of Radiation Shielding Properties of Glasses Contains PbO”, Journal of Measurement., pp. 1-12, 2018. [7] B. Yasser saddeek, “Ultrasonic investigations of some bismuth borate glasses doped with Al2O3”. Journal of Indian Academic Science., vol. 38, pp. 241-246, 2015 [8] G.V. Jagadeesha Gowda, and B. Eraiah, “Synthesis and Structural Studies Of Praseodymium Doped Silver Borate Glasses”. Journal of Engineering and Technology., vol. 1512, pp. 564-565, 2013. [9] A. Rani, R. Parmar, “Structural, Physical and Optical Study of Calcium modified bismuth borovanadate glasses: V2O5-B2O3-Bi2O3-CaO”. Journal of optical materials., vol. 143, pp. 114-135, 2023. [10] T. Sumathi and A.N. Kannappan, “Ultrasonic Investigation on Sodium and Calcium Tungsten Phosphate Glass System”. journals of chemical sciences., vol. 2(9), pp. 14-17, 2012. [11] M.A. Sidkey, M.S. Gaafar, “Ultrasonic studies on the network structure of ternary TeO2–WO3–K2O glass system”, Journal of Physica B., vol. 348, pp. 46–55, 2004. [12] N. Gera, M. Hussain, “Highly Stable Binding Proteins Derived from the Hyperthermophilic Sso7d Scaffold”, Journal of Molecular Biology., vol. 409, pp. 601-616, 2011. [13] A.M. Hafiz, “Prevention of contrast-induced acute kidney injury in patients with stable chronic renal disease undergoing elective percutaneous coronary and peripheral interventions: Randomized comparison of two preventive strategies”, journals of Catheterization and cardiovascular interventions., vol. 79, pp. 929–937, 2012. [14] E. Eren and S. kurama, “Characterization of porosity and defect imaging in ceramic tile using ultrasonic inspections”, Gazi University Journal of Science., vol. 38, pp. 2145-2151, 2012. [15] Y.B. Saddeek et al.,“FTIR and physical features of Al2O3–La2O3–P2O5–PbO glasses”, journal of non-crystalline solids., vol. 387, pp. 30-35, 2014. [16] A. Gaafar et al., “Effect of Green and Degree of Roasted Arabic Coffee on Hyperlipidemia and Antioxidant Status in Diabetic Rats”. Journal of Current Applied Physics., vol. 5(5), pp. 619-626, 2013. [17] T. Sumathi et al., “Protective effect of bacopa monnieraon methyl mercury-induced oxidative stress in the cerebellum of rats”. journal of Molecular Neurobiology., vol. 32, pp. 979-987, 2012. [18] N. Sangeetha, Ravibaskar, “Study on Physical and Elastic Properties of Lead Borate Glasses using Ultrasonic Techniques and Thermal Analysis”. International Journal for Scientific Research & Development., vol. 5, pp. 2321-0613, 2017. [19] A . A . Alemi, L.A. Kafi, “Preparation and characterization of terbium oxide doped sodium tetraborate glasses”. journal of SID., vol. 16, pp. 614-620, 2009. [20] R. Amaravel, “Structural and thermal properties of B2O3-Bi2O3-CaO glasses”. Journal of Advanced Physical Sciences., vol. 10, pp. 137-142, 2017. [21] S. G. motke, S.P. yawale, “Infrared spectra of zinc doped lead borate glasses”. Bulletin of material science., vol. 25, pp. 75-78, 2002. [22] C.Feller, “carbon sequestration in tropical areas general considerations and analysis of some endemic determinations for lesser antilles solis”. journal of Phys. chem.Glasses., pp. 19-31, 2001.
Copyright
Copyright © 2024 Sumathi T, Soundarraj C, Parkavi S. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66018
Publish Date : 2024-12-19
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

